Abstract
Background: Tirabrutinib (TIR) is a second-generation oral Bruton's tyrosine kinase (Btk) inhibitor that is more selective for Btk than first-generation Btk inhibitors such as ibrutinib. TIR has been designed to improve the safety and efficacy of ibrutinib, and has been suggested to have a more favorable toxicity profile, including in atrial fibrillation (Af) and bleeding events. In 2020, TIR was approved in Japan for use in treatment-naïve (TN) or relapsed/refractory (R/R) Waldenström's macroglobulinemia (WM) based on the results of a phase II study (trial registration: Japic CTI-184057). In the phase II study, 18 TN (cohort A) and 9 R/R (cohort B) WM patients were enrolled. We previously reported the results of this study with data cutoff on August 28, 2019 (Sekiguchi et al., Cancer Sci. 2020 Sep; 111 (9): 3327-3337). In the report, major response rate (MRR, ≥ partial response [PR]) was 88.9% in cohort A and 88.9% in cohort B. Overall response rate (ORR, ≥ minor response [MR]) was 94.4% in cohort A and 100% in cohort B, with median follow-up period of 6.5 months (cohort A) and 8.3 months (cohort B). We herein report two-year follow-up data of this study (data cutoff on February 1, 2021).
Methods: Phase II study was an open-label, prospective, single-arm study conducted at 19 facilities in Japan. TN or R/R WM patients with serum IgM ≥ 500 mg/dL were treated with TIR 480 mg under fasting conditions once daily until disease progression or clinically unacceptable toxicity. The primary endpoint was MRR assessed by an independent review committee (IRC) according to the VI th International Workshop on WM (IWWM) criteria. Secondary endpoints included ORR, time to major response (TTMR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
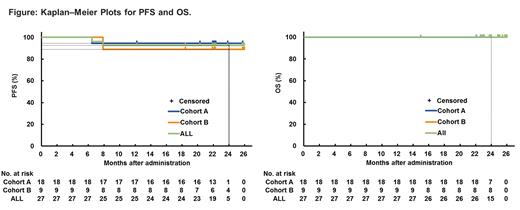
Results: The median follow-up period was 23.8 months in cohort A and 25.4 months in cohort B. Of the 27 patients, 5 patients discontinued treatment due to adverse events (1 patient with atypical mycobacterial infection), progressive disease (1 patient), physician's discretion (1 patient), or withdrawal by subject (2 patients). IRC-assessed MRR was 94.4% (95% CI: 72.7-99.9) in cohort A and 88.9% (95% CI: 51.8-99.7) in cohort B. IRC-assessed ORR was 94.4% (95% CI: 72.7-99.9) in cohort A and 100.0% (95%CI: 66.4-100.0) in cohort B. The rate of ≥ very good PR was 33.3% in both cohorts. Median TTMR was 1.9 months (range 1.0-20.3) in cohort A and 2.1 months (range 1.0-3.7) in cohort B. Median PFS, OS and DOR were not achieved in either cohort. Two-year PFS rate was 94.4% in cohort A and 88.9% in cohort B. Two-year OS rate was 100% in both cohorts. Continued reductions in IgM were observed in patients who remained on treatment. In contrast, 3 patients who discontinued treatment for reasons other than withdrawal by subject had an increase in IgM after discontinuation. In the overall study population, the most common adverse events (AEs) were rash (44.4%), neutropenia (33.3%), and nasopharyngitis (25.9%). Two patients experienced Af and 9 patients experienced bleeding AE. All events of rash, nasopharyngitis, Af and bleeding AE were < grade 3. The most frequently reported ≥ grade 3 AEs were neutropenia (22.2%), lymphopenia (18.5%), and leukopenia (11.1%). During this extended follow-up period, no new ≥ grade 3 treatment-related AEs were observed except for hypertriglyceridemia observed in 1 patient.
Conclusion: TIR demonstrated sustained efficacy in TN and R/R WM patients during the two-year follow-up period. Additionally, no new safety signals for TIR were identified compared to previous reports. TIR is a useful treatment option for WM.
Suzuki: Abie: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; ONO: Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Sekiguchi: Ono: Research Funding; A2 Healthcare: Research Funding; Astellas: Research Funding; Janssen: Research Funding; Merck Sharp & Dohme: Research Funding; Otsuka: Research Funding; Pfizer: Research Funding; PPD-SNBL: Research Funding; Sumitomo Dainippon: Research Funding; Daiichi Sankyo: Research Funding; Bristol Myers Squibb: Research Funding. Rai: Janssen Pharmaceutical: Speakers Bureau; Ono Pharmaceutical: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau. Munakata: CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria, Research Funding; Takeda Pharmaceutical Company Limited.: Honoraria, Research Funding; Novartis Pharma K.K.: Honoraria, Research Funding; Janssen Pharmaceutical K.K.: Honoraria, Research Funding; ONO PHARMACEUTICAL CO., LTD.: Honoraria, Research Funding; Sanofi K.K.: Research Funding; Bristol-Myers Squibb K.K.: Honoraria, Research Funding; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Amgen inc.: Research Funding; MSD K.K.: Research Funding; Astellas Pharma Inc.: Research Funding; AbbVie GK: Research Funding; Kyowa Kirin Co., Ltd.: Research Funding; Celgene K.K.: Honoraria; SymBio Pharmaceuticals Limited: Honoraria; Eisai Co., Ltd.: Honoraria; AstraZeneca K.K.: Honoraria. Handa: Ono: Honoraria; BMS: Honoraria; Janssen: Honoraria; Daiichi Sankyo: Research Funding; Celgene: Honoraria, Research Funding; Chugai: Research Funding; Kyowa Kirin: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Abbvie: Honoraria; Shionogi: Research Funding; MSD: Research Funding. Shibayama: Otsuka: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Nippon Shinyaku: Honoraria; Fujimoto: Honoraria; Daiichi Sankyo: Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Research Funding, Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Ono: Research Funding, Speakers Bureau; Celgene: Research Funding; Mundi Pharma: Honoraria; Essentia Pharma Japan: Research Funding. Terui: AbbVie: Speakers Bureau; Celgene: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Esai: Speakers Bureau; Janssen: Speakers Bureau; MSD: Speakers Bureau; Ono Pharmaceutical: Speakers Bureau; Takeda Pharmaceutical: Speakers Bureau. Fukuhara: AbbVie: Honoraria; Bayer: Research Funding; Celgene: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Eisai: Honoraria; HUYA Bioscience International: Honoraria; Incyte: Research Funding; Janssen: Honoraria; Kyowa Kirin: Honoraria; Nippon Shinyaku: Honoraria; Novartis: Honoraria; Ono Pharmaceutical: Honoraria, Research Funding; Takeda Pharmaceutical: Honoraria; Zenyaku Kogyo: Honoraria. Tatetsu: Ono Pharmaceutical: Honoraria; Chugai: Honoraria; Eisai: Honoraria; Novartis: Honoraria; Mesoblast: Patents & Royalties. Iida: Chugai: Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Glaxo SmithKlein: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding. Iguchi: Ono Pharma USA, Inc.: Current Employment. Izutsu: Allergan Japan: Honoraria; Symbio: Honoraria, Research Funding; Pfizer: Research Funding; Ono: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; MSD: Research Funding; Janssen: Honoraria, Research Funding; Incyte: Research Funding; Genmab: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Beigene: Research Funding; Bayer: Research Funding; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Yakult: Research Funding; Takeda Pharmaceutical: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; HUYA Bioscience International: Research Funding; Eisai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; FUJI FILM Toyama Chemical: Honoraria.
Tirabrutinib. Clinical trial for WM/LPL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal